Our Services
Please click the +-sign to read more.
• Chemical Industry
Determination of chemical exposure over time in humans as a basis for risk assessment
The difficulty in determining the exposure of electrophilic compounds and metabolites lies in their inherent reactivity. Structural modifications on blood proteins and nucleic acids (known as adducts) from these electrophiles can serve as valuable biomarkers for assessing chemical exposure from both exogenous and endogenous sources. Therefore, measurement of the adducts can be used as biomarkers of exposure to specific electrophiles in the general population or targeted groups. For instance, adducts to hemoglobin have applications in epidemiological studies and in estimating occupational exposure to electrophiles.
Why measure electrophilic compounds?
- Component of the exposome – stem from exogenous and endogenous sources
- React with DNA and proteins – risk for genotoxic, immunogenic and other effects
- Molecular epidemiology – quantified risk assessment of occupational and environmental exposure to identified chemicals.
Advantages of hemoglobin (Hb) adducts as biomarkers
- Electrophiles are reactive and unstable, hence direct measurement is not optimal.
- Hb adducts of electrophiles can be measured after a relatively long period.
- Hb half-life is ~63 days in humans.
- Different electrophiles bind to N-terminal valine (Val) of Hb1 – allowing their characterization and measurement by Hb adducts
Our service
We employ high-resolution mass spectrometry (HRMS)-based methods to analyze adducts to hemoglobin. This includes applying the FIRE method,1,2 which is a development of the modified Edman degradation – the adduct is derivatized and released with the N-terminal valine tag. The corresponding analyte specific to the electrophile is enriched by SPE and quantified by LC-HRMS. HRMS data combined with expert knowledge is used for profiling and characterization of the adducts. Depending on the research question, analyses can be conducted across various species and tissue types.
[1] von Stedingk H, et al. A new modified Edman procedure for analysis of N-terminal valine adducts in hemoglobin by LC-MS/MS. J Chromatogr B. 2010.
[2] Vryonidis E, et al. 2024. Estimation of intake and quantification of hemoglobin adducts of acrylamide in adolescents in Sweden. Front Nutr. 2024.
• In vitro Metabolism
The lead optimisation phase often includes soft spot Met ID in human and toxicity or pharmacological species to understand metabolic instability. When choosing a candidate drug to progress through development phase, generation of a more complete in vitro cross species Met ID data set is crucial. It is the best tool at this stage to understand similarities and discrepancies between human and toxicity species, in terms of metabolic pathways and as step 1 in the “Metabolites in Safety Testing” evaluation.
We have core expertise in performing in vitro Met ID and metabolic stability (in vitro CLint) designed for candidate drug selection or on candidate drugs prior to FTIM.
We also know how to evaluate existing in vitro data and advice on next step how to proceed mitigating risks of having a MIST issue later on.
The in vitro metabolic stability and Met ID can be performed in different metabolic systems, species and gender depending on the question.
- Metabolic Stability
Metabolic stability in vitro (in vitroCLint) data can be used to evaluate how quickly a candidate drug will be eliminated by enzymes through biotransformation.
Identifying candidates with favourable pharmacokinetics (which is a result of, e.g., metabolism) early in discovery helps accelerating development, reduce costs, and support risk-based decision making. Metabolic stability studies are also a valuable tool for optimizing species selection in non-clinical studies and improving predictability in humans. - Metabolite Identification (Met ID)
Met ID plays a crucial role at multiple stages of drug discovery and development. In discovery in vitro Met ID enables soft spot identification and after candidate selection a cross-species comparison is required prior to FTIM to among other reasons support the selection of appropriate toxicity species and studies. The results might trigger non-clinical in vivo Met ID to confirm if species discrepancies are true or due to in vitro phenomena (lack of certain enzymes etc).
The systems used are liver microsomes, S9 and/or cryopreserved hepatocytes. For specific questions there are microsomes and S9 available for other tissues as well, e.g. skin. For each system and biotransformation of interest relevant cofactors are used.We perform in vitro Met ID designed for candidate drug selection or on candidate drugs prior to FTIM. U/HPLC coupled to high resolution mass spectrometry (QE-Orbitrap-MS) is majorly used, which may be supported by additional detection techniques such as LC-UV if required. Data processing is performed by software-aided data mining combined with expert knowledge in biotransformation.We also provide interpretation of the data, identification of potential issues going ahead and may suggest what could be the next step.
- Recombinant Enzymes
Recombinant enzymes (Phase I and Phase II) assays provide information into which drug-metabolizing enzymes are responsible for the metabolism of a compound. These studies can also support in vivo data and support DDI investigations, offering a clearer understanding of metabolic pathways and potential safety risks.In addition to cytochrome P450 (CYP) enzymes, we perform reaction phenotyping studies to evaluate other drug-metabolizing enzymes that may be important to investigate, such as non-CYP oxidases (FMO, MAO, AO), carboxylesterases, uridine 5′-diphospho-glucuronosyltransferases (UGT), sulfotransferases (SULT) as well as sulfatase (STS). - Reactive Metabolite Assessment
Unexpected drug toxicity can arise from reactive metabolites that bind to proteins or DNA. Because of their short-lived nature, these metabolites are stabilised with trapping agents such as GSH, KCN, methoxylamine, guanosine or SCA before analysis. The trapping is performed in S9 or microsomal incubations fortified with the trapping agent and relevant cofactors.Using LC-HRMS, we deliver accurate, high-quality data to characterise reactive metabolite formation. - Acyl Glucuronides Investigation
Acyl glucuronides formed from carboxylic acid-containing drugs or as a result of carboxylation and glucuronidation may cause adverse drug reactions through covalent protein binding. The risk assessment of acyl glucuronides are among others based on the stability of the acyl glucuronide, e.g. the rate/ability of acyl migration. Our assays support the investigation of acyl glucuronide formation and stability, to support the assessment of the risk of toxicity associated with these potentially reactive metabolites during drug development.
• Metabolite profiling and characterisation in non-clinical species
Met ID in non-clinical species early on can be performed to confirm or retract potential discrepancies between species in vitro. The plasma metabolite profile (circulating metabolites) many times reflect the metabolic pathways in vivo to a larger extent than in vitro data despite the fact that not all metabolites formed and excreted are present at all or at detectable levels in plasma.
We perform in vivo Met ID studies as part of MIST evaluation but also exploratory and as problem solving activities. We handle blood, plasma, urine, feces, bile, tissues and other body fluids (e.g. saliva, humour).
• Human metabolite profiling and characterisation (circulating and excreted metabolites)
The most important data is Met ID in vivo in human. First available samples comes with the SAD study. It is a single dose but still very useful particularly if there is a need for data as soon as possible. Sometimes also from a dose perspective SAD samples are more useful. In addition to plasma, urine Met ID is an important tool for some compounds to get a better overview of the metbolic pathways and often supports the Met ID in plasma where lower levels might be seen.
From MIST perspective repeated dose studies are used where the metabolite profile in MAD samples “set the scene” of which metabolites require adequate exposure in toxicity species.
We perform Met ID in FIH samples. We can guide you through the need for your project.
• MIST analysis (exposure comparison between human and non-clinical safety species)
The exposure comparison of human metabolites between human and toxicity species is performed to understand if human metabolites are adequately exposed in the toxicity species.
To perform and evaluate exposure comparison in the best way several aspects have to be considered.
We know MIST analysis and perform exposure comparison utilising samples from repeated dose studies. We can guide you through the best approach for your project.
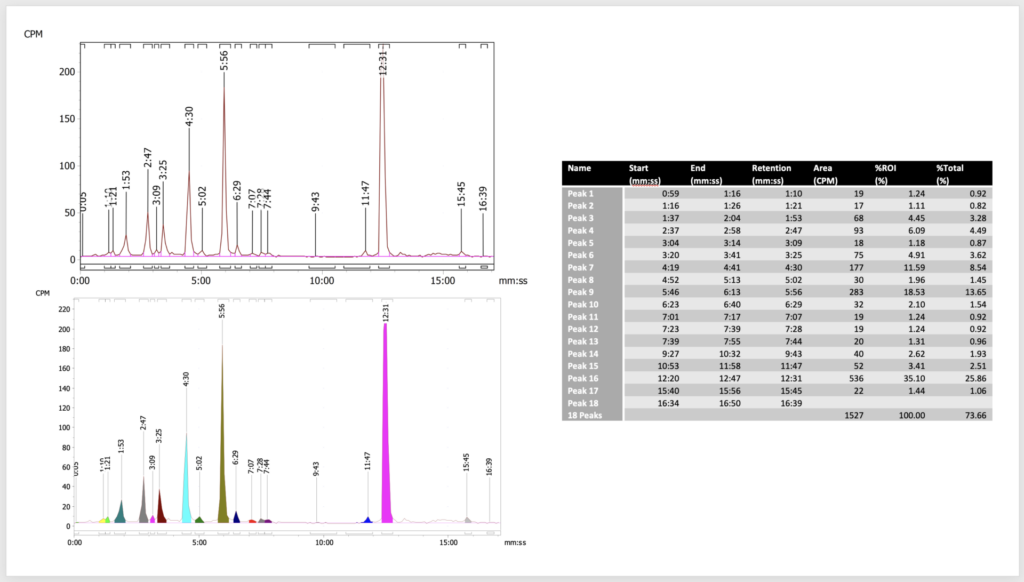
• Radiolabel-assisted metabolite profiling and characterisation in samples from human and preclinical A(D)ME studies
Radiolabel-assisted metabolite profiling is important to achieve a more quantitative understanding of the metabolism and excretion than is possible with other detection methods.
As experts in radiolabel-assisted metabolite profiling and characterisation we are aware of all the pitfalls related to such applications.
We can support you in:
- advice on labelling position from a metabolic perspective in collaboration with radiochemists
- advice on concentrations in vitro, doses in vivo including specific activities required to obtain useful data
- LC-MS method development suitable for both on-line and off-line radioactivity monitoring (RAM)
- recovery-based sample preparation development for any biological matrix
- recovery determinations
- metabolite profiling using on-line (parallel coupled) RAM or fraction collection to 96-well LUMA plates with offline RAM in TopCount.
- metabolite characterisation using HRMS based on radiochromatograms
- MS/RAM response factor determinations in any matrix/sample
- radioactivity determination in organs from animals dosed with 14C- or 3H-compounds.
- outsourcing in life ADME studies
- Full Met ID in samples from A(D)ME study performed in any species (rodent, non-rodent, or human)
Against this we can support you in any Met ID study or problem-solving activity utilising 14C or 3H.
• Non-GLP bioanalysis

